![Side view of Earth's atmosphere taken from the International Space Station [NASA]](img/aerobanner.jpg)
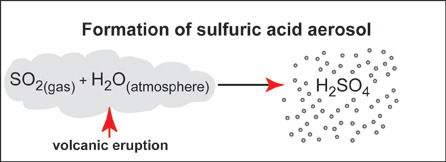
Some of the gases that are given off during volcanic eruptions undergo reactions in the atmosphere to form aerosols. In particular, SO2 gas reacts to form tiny droplets of sulfuric acid that can have important effects on the atmospheric radiation budget.
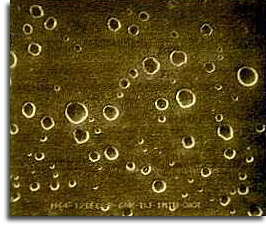
The image here shows sulfuric acid aerosol particles collected in the atmosphere following the 1982 eruption of El Chichon volcano, Mexico. The largest droplets are 1 micron in diameter.
Aerosols are a mixture of very small liquid droplets and/or solid particles with air. The most important volcanic aerosol consists of droplets of sulfuric acid in the atmosphere. The droplets, usually sub-micron to micron size in diameter (1 micron is equal to 1/10,000 of an inch!), are formed by a chemical reaction of SO2 gas and water in the atmosphere (see below).
Because of their small size, volcanic aerosols can remain in the atmosphere for several years following an eruption. They are light in color, so the main impact of an aerosol layer is to block incoming solar radiation, reflecting it back out into space.
The chemical reaction to convert sulfur dioxide gas to sulfuric acid droplets occurs in three major steps:
1. SO2 (gas) + OH → HOSO2
2. HOSO2 + O2 → SO3 + HO3
3. SO3 + H2O → H2SO4 (liquid)