The discharge of sulfur into the atmosphere during volcanic eruptions results in the formation of sulfuric acid droplets.

The amount of sulfur given off, or degassed, during an eruption can be determined from the difference between the amount of sulfur in the magma before the eruption and the amount remaining in the erupted rocks or volcanic ash once the eruption has occurred. It is also necessary to know the total volume of magma erupted in order to make the calculation.
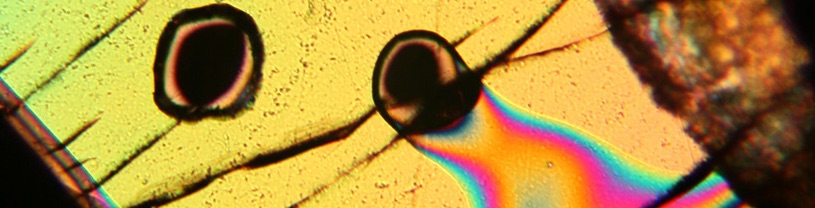
The amount of sulfur in a magma prior to eruption can be determined by analyzing magma inclusions that are trapped in crystals that form in the magma deep within the Earth. Magma inclusions do not lose their sulfur during an eruption because they are sealed off by the host crystal before degassing happens. The magma inclusion in the photo here is only about 0.08 mm in diameter! It is made of glass.

An electron microprobe can be used to measure sulfur and many other elements in volcanic rock samples. The instrument usies a beam of electrons to analyze a spot on a sample less than 0.01 mm diameter. In this exercise, you will learn more by simulating some electron microprobe measurements of magma inclusions from the Laki eruption.
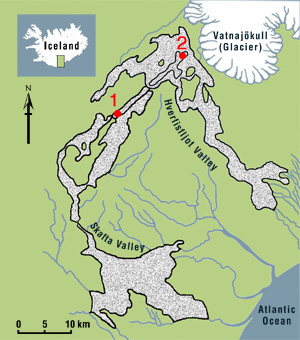

1. Click on each of the two sample localities shown on the map (red dots 1 and 2).
2. Carry out the electron microprobe analysis of magma inclusions in the pop-up window for each sample.
3. Record the results in your field notebook.
When you have completed your microprobe analyses, you are ready to move ahead to the next step.