
One of the most important properties of magma is viscosity, or the resistance of magma to flow when subjected to a force.
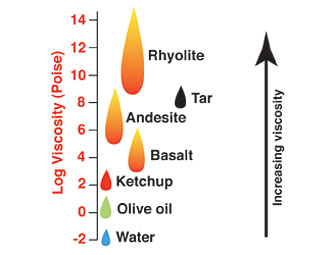
Viscosity is measured in the units of Poise and there is a tremendous range displayed by magmas erupted on Earth.
In general, the viscosity of magma increases as the silica content increases from basalt to rhyolite.
Magmatic viscosities are much larger than fluids that we are familiar with, such as water or olive oil (see the figure here for comparison).
Press the button to launch a module that will let you examine the viscosities of different fluids and magmas.
Viscosity Simulator →The large range of magmatic viscosities is related to differences in the structure of magmas at the molecular level, which varies as a function of silica content. Magmas are fluids but they possess structure in the form of silicon-oxygen building blocks, that are linked together to form tetrahedra.
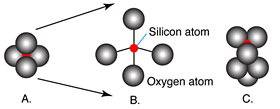
Magmas that are rich in silicon form many linked tetrahedra that result in the polymerization of the liquids.The figure here shows the structure of a silica tetrahedron (A and B) and the linkage of two tetrahedra by sharing an oxygen (C).
The bonds that form between shared oxygen atoms are strong, thus giving the liquid strength and high viscosity. Lower silica magmas contain more cations such as Ca, Mg and Fe that form weaker bonds and inhibit the development of strongly linked silica tetrahedra. Therefore, low-silica magmas have a lower viscosity.
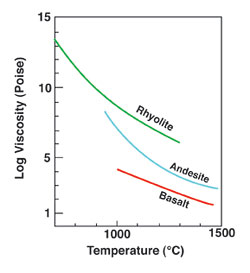
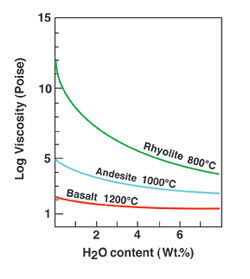
Magma viscosity is also influenced by the temperature and gas content. Decreasing temperature results in a dramatic increase in viscosity (see plot of viscosity vs. temperature). Thus, as magma is erupted at the Earth's surface, it immediately starts to cool and becomes more viscous.
Dissolved gases such as water and carbon dioxide tend to decrease the amount of polymerization in magma, resulting in a decrease in viscosity compared to gas-free magma (see plot of viscosity vs. H2O).